
Strangles
A page about strangles in horses describing cause, clinical signs, diagnosis and control.
Introduction
Strangles is a highly contagious infection, affecting all equidae including horses, donkeys and ponies. Strangles is the common name given to the bacterial infection of the respiratory tract which is characterised by abscessation of the lymph nodes in the head and neck. It is one of the most frequently diagnosed respiratory infections in horses worldwide and it has been described in horses for over 800 years. This infection has the
potential to cause major economic losses to the equine industry both within Ireland and worldwide due to its prolonged course, extended recovery period and associated serious complications, including possible
fatalities. Recent research has highlighted the extent of the problem within Ireland, estimating that over 40% of horses in Ireland have been exposed to this disease and may be chronic carriers of infection. Due to the nature of the equine industry, with widespread movement of horses for sales, breeding and competition, the potential for spread of this contagious
disease is immense.
Aetiology (Cause of Disease)
Strangles is caused by the bacterium Streptococcus equi subspecies equi; a member of the Lancefield Group C Streptococci.
Epidemiology (Factors Influencing Occurrence of Disease)
Strangles is the most common bacterial respiratory infection of horses. In a susceptible population, strangles occurs most frequently in horses up to 5 years of age. Foals born to immune dams are usually protected by colostrum derived antibodies up to the age of approximately 3 months. The majority of horses will recover fully from the infection however the disease may be prolonged and can cause considerable suffering to the affected horse. It is estimated that up to 10% of horses may die due to complications of severe infection. Furthermore up to 10% of affected animals may develop a chronic carrier state which results in intermittent shedding of the contagious bacteria. Infection can spread rapidly through a population of horses particularly
where horses are housed in close proximity to each other, for instance a stable yard. Morbidity of up to 100% can occur in an exposed naïve herd. Disease spreads quickly and due to the development of the carrier state infection can persist in a yard for many months or even years. Disease can be spread by direct contact with nasal, ocular or lymph node secretions from infected horses. Indirect transmission may also occur via contaminated housing, water sources, feed or feeding utensils, equipment,
and tack. The clothing of handlers, caretakers, farriers, and veterinarians may also play a role in the transmission of disease. Shared drinking bowls have been identified as a common reservoir of the infection. It is thought that the bacteria could survive in water for several weeks.
The host becomes infected when bacteria attach to tonsillar epithelium and lymph tissue after inhalation or ingestion of infected secretions. From this location the bacteria move to the surrounding lymph nodes of the head and neck. Due to the presence of the bacteria and the subsequent immune response, abscesses form in the affected lymph nodes. The incubation period for strangles is typically 3-14 days, with abscesses formed from up to 2 weeks after initial infection.
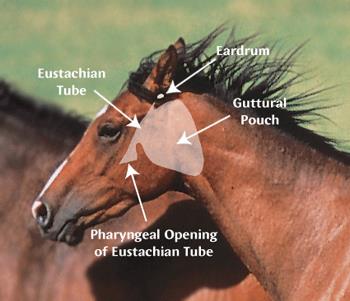
Occasionally the bacteria may travel via the lymphatic system or blood vessels to other lymphoid tissue or other organs in the body. The Guttural pouches may also become infected if the adjacent retropharyngeal lymph nodes rupture. Guttural pouch infections may also occur when infected material drains into the pouches from the pharynx. Rarely, lymphatic or haematogenous spread of bacteria occurs during the early, febrile stage and results in “bastard strangles”. Bastard strangles refers to the abscessation of lymphoid tissue or other organs beyond the head and neck. Disease prevalence is generally considered to be much higher than official reports. The Irish Equine Centre diagnostic laboratory cultures the causative organism from many new outbreaks annually. Established outbreaks may last for months or even years particularly in large horse populations with frequent new arrivals that provide a supply of susceptible animals.

Clinical Signs
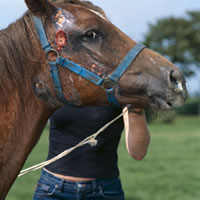
Typically a high temperature (103-106℉) will be the initial presenting sign of infection. Anorexia, lethargy, depression and a nasal discharge develop 24-48 hours after the rise in temperature. The nasal discharge is initially serous but quickly becomes purulent. With progression of disease the lymph nodes in the submandibular and retropharyngeal (neck) regions become hard and painful. Difficulty swallowing and breathing may occur due to the enlargement of these lymph nodes and the horse may stand with an outstretched neck to assist breathing and relieve lymph node pain. Often these lymph nodes rupture releasing a thick purulent heavily contaminated discharge. In uncomplicated cases horses recover one to two weeks later.
Some lower virulence strains of S. equi may cause milder clinical signs particularly in older horses. These strains result in milder clinical disease with or without rupture of the lymph nodes. Complications of Strangles have been estimated to occur in approximately 20% of all cases. These complications include:
- Purpura haemorrhagica may develop in a small number of horses 2-4 weeks after Strangles. Purpura haemorrhagica is caused by an immune-mediated hypersensitivity response to Streptococcus equi ss equi antigens that results in widespread vasculitis (inflammation of the blood vessels). The clinical signs of this disease are swelling of the limbs and underbelly, small haemorrhages on the mucosal surfaces of the lips, vulva etc., depression, pain and fever. Intensive therapy along with supportive care is required for horses affected by this condition.
- Bastard strangles describes the spread of infection to sites in the body other than the lymph nodes of the head and neck. In this condition abscesses in distant organs and lymph nodes may grow and later rupture leading to possibly fatal complications. Most internal S. equi abscesses are located in the abdomen or thorax, although involvement of other locations has been reported, including cases of brain abscesses associated with S. equi infection. Bastard strangles may occur in up to 10% of affected horses.
- Guttural pouch empyema is one of the most common complications of strangles, and results from extension of infection into one or both guttural pouches. Typically both guttural pouches will be affected but occasionally it is unilateral. Affected horses may display chronic mucopurulent nasal discharge and often increased respiratory noises due to impeded airflow secondary to the enlarged pouches. Infection may then persist in the guttural pouches and these affected animals may become persistent shedders of infection. These persistent shedders may also show no clinical signs of disease but may act as a source of infection for susceptible animals.
- A wide range of other clinical conditions have been described as rare sequelae to infection including bronchopneumonia, myocarditis, septicaemia in foals, glomerulonephritis, and even septic arthritis.
Diagnosis
Diagnosis is initially based on recognition of common clinical signs including pyrexia, lethargy, anorexia, nasal discharge, enlarged painful lymph nodes and eventual lymph node rupture. A more definitive diagnosis is based on the isolation of Streptococcus equi in the affected horse. Swabs taken from the nasopharynx, guttural pouch and from abscesses are cultured to confirm the clinical diagnosis. Recent studies suggest that S. equi is a subtype of S. zooepidemicus. It is however necessary to differentiate these bacteria for the purpose of control of disease. On blood agar both are haemolytic however S. equi is described as being encapsulated. Typically this gives the S. equi colonies a characteristic honeydew, mucoid appearance. However some clinical isolates can appear indistinguishable from S. zooepidemicus on agar. In these cases, differentiation of S. equi from S. zooepidemicus depends on testing for the ability to ferment lactose, sorbitol and trehalose. A negative result in all of these tests is taken as an identification of S. equi.
The organism may also be identified by PCR testing. Polymerase chain reactions (PCR) tests can amplify a segment of DNA to detectable amounts so that highly sensitive S. equi detection is feasible. PCR may even detect dead organisms draining into the pharynx from the guttural pouches. As this test is also sensitive to dead organisms some horses may remain PCR positive for many months. In this case it is advisable to confirm an active infection by culture. An antibody test is also available for diagnosing recovered cases. This test has been used to estimate the levels of exposure to Strangles within populations of horses. Some other diagnostic techniques used by veterinarians are radiography, guttural pouch endoscopy, and lymph node ultrasonography.
Control
In the event of an outbreak, isolation is key to prevent the spread of infection. All exposed and clinically ill horses should be isolated for a period of 4 weeks if possible. Avoid indirect transmission by using dedicated equipment and work wear for isolated animals. Advise personnel about disinfection techniques and minimising traffic to and from the isolation area. Isolated horses should have three negative nasopharyngeal swabs or guttural pouch washes taken at weekly intervals before they are reintroduced to the herd. Culture of guttural pouch washes collected endoscopically may be more sensitive in picking up potential carrier horses.
Maintain a closed yard until the infection is controlled. Ideally no horses should enter or exit the farm until outbreak is over. Disinfect areas and equipment that have been used by infected and suspect horses, including stalls feeders and remove as much organic waste materials as possible.
Perform regular rectal temperature checks (at least every 12 hours) as feverish animals can be detected and removed from cohort animals before they begin shedding bacteria. Regular clinical checks are also useful to identify cases early. Identify the carriers of infection and treat or remove from the herd.
To prevent the introduction of disease to a naïve herd, newly acquired horses should be isolated for 4 weeks before introduction. In areas of risk all newly acquired horses should have a complete and up to date vaccination history before introduction to the herd. Occasionally horses are swabbed before introduction to the herd although some horses can give rise to false negative results. Good hygiene and biosecurity in the yard are vital to minimise infections. Avoid stressors like overcrowding. Maintain
disinfection of equipment and stables between horses. If travelling to events minimise mixing of horses from different yards and avoid sharing equipment with other horses Vaccination in conjunction with strict hygiene and biosecurity management
can form a vital part of a comprehensive control programme. Vaccination is recommended if there is a risk of introduction of disease due to contact with horses from areas where this pathogen is known to be present. A live vaccine is available to reduce clinical signs and the occurrence of lymph node abscesses in exposed horses. It is recommended that all horses stabled together are vaccinated. It is advisable that you would consult with your veterinary practitioner before using the vaccine, to discuss the risk profile for your horse/horses and a possible vaccination programme. For further information on strangles disease and vaccination in horses please click here.

Treatment of Clinical Cases
International scientific opinions differs with regard to the treatment regimes for Strangles infections. It is agreed however that the treatment plan for Strangles infection should vary depending on the stage of the infection and clinical signs. For this reason treatment plans are best designed in conjunction with your veterinarian. In early cases of disease the vet may recommend antibiotic therapy or supportive care. Occasionally the veterinarian may decide to lance affected lymph nodes to ease the pain
experienced by the horse. In more complicated cases of strangles painkillers or steroids may be required. Endoscopic guided procedures may also be performed particularly for cases of guttural pouch empyema. There is no single treatment protocol for strangles; it is therefore wise to consult your veterinarian for advice on the most suitable treatment options.
