
Diabetes Mellitus
A page about canine diabetes mellitus describing cause, clinical signs, diagnosis and control.

Introduction
Diabetes mellitus is a fairly common endocrine disorder of dogs and cats. It is commonly seen in middle aged to older pets.
Diabetes mellitus is characterized by a relative or absolute insulin deficiency. This results in an inability to transport glucose from the bloodstream into cells. Blood glucose concentrations are elevated. If the renal threshold is exceeded, glucose is excreted in the urine, leading to polyuria and polydipsia. In addition, glucose metabolism is impaired which leads to a deterioration in the general condition of the animal and, if untreated, to death.
Aetiology
Diabetes mellitus can be classified as follows:
Type 1
· Occurs due to immune-mediated destruction of the insulin-producing (beta, ß) cells of the pancreas
· Predominant form of primary diabetes mellitus in dogs
· Possible genetic component in dogs
· Results in total absence of insulin production, cases (i.e. all diabetic dogs) with this form of diabetes mellitus must be treated with insulin
Type 2
· Cause not well defined
· Characterised by obesity (animals may no longer be overweight at the time of presentation), loss of tissue sensitivity to insulin, and deposition of amyloid in the pancreatic islets
· The pancreas may be producing none, too little, normal amounts, or even excessive amounts of insulin, but this insulin is failing to achieve its normal action
· The pathological basis of this “transient diabetes mellitus” is yet to be fully determined.
· There is currently no way of predicting whether diabetes mellitus will be transient or permanent at the time of diagnosis.
· Rare in dogs
Type 3
This is where diabetes mellitus occurs secondary to another disease, such as:
– Cushing’s disease (hyperadrenocorticism) in dogs
– Acromegaly
– Drug treatment (e.g. corticosteroids, progestagens)
– Extended use of these drugs may result in permanent diabetes mellitus
– Generalised disease of the pancreas, such as that caused by an invasive tumour or chronic pancreatitis, if a sufficient proportion of beta cells are destroyed.
– The underlying condition should be treated and, if necessary, the diabetes mellitus
Epidemiology
In the 1960’s, estimates of diabetes 1:152 in dogs. In the 1980’s, the incidence was reported to be 1:100 to 1:2000 in dogs. More recently the reported incidence is around 1:500 (0.2%) in both dogs and cats.
Diabetic dogs usually range in age from 4 -14 years (peak incidence 7-9 years).
In dogs a higher incidence is seen in (entire) bitches. Intact bitches should be spayed as soon as possible, ideally before insulin treatment has been started as this removes concerns about the development of hypoglycaemia.
Certain breeds of dog appear to have a higher risk of developing diabetes mellitus: Keeshond, Puli, Miniature pinscher, and Cairn terrier. There is also possibly a higher risk in Poodles, Dachshunds, Miniature Schnauzers and Beagles.


Clinical Signs
The classical signs of uncomplicated diabetes mellitus, in dogs, are
· Polyuria/polydipsia (passing excessive amounts of urine / drinking too much)
· Polyphagia (eating too much)
· Rapid weight loss in spite of a good appetite
· Increased susceptibility to infection (e.g. of the urinary tract)
The complications of diabetes mellitus that are seen in dogs are:
· Cataracts
· Diabetic ketoacidosis (DKA)
These animals have often been ill for only a short time. A variety of signs including severe depression, anorexia, vomiting, oliguria (reduced volume of urine) or anuria (not passing urine) and hyperventilation may be observed. These animals are often in shock and may have a distinct smell of acetone on their breath.
Hyperosmolar nonketotic syndrome: These animals have often been ill for longer than animals with DKA. Animals with hyperosmolar syndrome have extremely high blood glucose concentrations without ketoacidosis. These animals are usually very lethargic or comatose.
Diagnosis
Diagnosis can be based on:
a) Clinical Signs
The clinical findings are non-specific. The physical findings may include
· Lethargy
· Dehydration
· Unkept hair coat and dehydration
b) Laboratory tests
In dogs a diagnosis of Diabetes Mellitus is based on the clinical signs and the presence of a persistent fasting hyperglycaemia and glucosuria.
The normal reference range for blood glucose in dogs is 3.9 to 5.0 mmol/l (70 to 90 mg/dl)
Repeated fasting concentrations of glucose that are persistently >7.5 mmol/l (135 mg/dl) in the dog, indicates diabetes mellitus. If the blood glucose concentrations are consistently in the upper reference range, 6.0-7.5 mmol/l or 108-135 mg/dl, a glucose tolerance test may be needed to confirm the diagnosis.
The reference range for fructosamine varies from laboratory to laboratory, however the following can be used for guidance:
· In dogs: 258-343 µmol/L
The renal threshold for glucose is a blood glucose concentration of around:
· In dogs: 10 mmol/l (180 mg/dl)
If the blood glucose level exceeds this threshold, glucose is excreted in the urine.
Control
The aim of insulin therapy in pets is to attain reasonable control of the clinical signs of diabetes mellitus and blood glucose concentrations by using once or twice daily injections of medium to long acting insulin preparations. A novel device for administering insulin to pets has been introduced to the Irish market by MSD Animal Health. Further information on the use of this device, called Vetpen, is available by clicking here.
As there is individual variation in the response to given insulin, an initial trial or stabilization period is usually undertaken. The initial stabilization period can last for up to 6 months. The owner should continue to monitor their animal at home, by either checking blood or urine glucose concentrations. As each animal’s insulin requirements change with time, periodic rechecks are essential, even after the initial stabilization period. These rechecks should include a clinical examination plus the construction of a blood glucose curve and some additional laboratory tests.
If a dog is in crisis or in a coma, it should first be treated with fluids and intravenous insulin.
It should be understood that it is impossible to maintain a normal glucose concentration throughout the entire day. If the blood glucose concentration can be maintained between 5 and 15 mmol/l (90 and 270 mg/dl) during a substantial part of the day, the clinical signs disappear and the main aims of therapy will have been achieved.
Dose adjustment
Dose adjustment should be based on biochemical assessment of the glycaemia. There are two body fluids that can be sampled to assess glycaemic control.
· Urine
· Blood
Urine glucose monitoring
Many vets recommend urine glucose testing as a method of monitoring diabetes mellitus in dogs. This method is simple and inexpensive but it has some serious limitations that must be understood and taken into consideration.
This is based on the fact that once the blood glucose concentration exceeds the renal threshold; glucose is spilled into the urine. The amount of glucose present in the urine depends on how high the blood glucose was, and how long the blood glucose was high. In general, urine testing is more useful in dogs than in cats.
Blood glucose monitoring
This is a way of testing how much glucose is in the blood. There are a number of ways of assessing current blood glucose concentrations.
· Collecting a blood sample in a tube containing fluoride as an anticoagulant and submitting this to a laboratory for analysis. Plasma glucose concentrations are measured (“gold standard”).
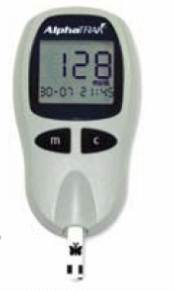
· Collecting a drop of blood from the ear (pinna) or a carpal pad or a footpad and analyzing this using a hand-held blood glucose meter. A small test strip with the drop of blood on it is by inserted into a small machine (hand-held blood glucose meter), which reads the strip and shows the blood glucose level in a digital display window. The drop of blood can be produced using a sterile needle or a special lancet (razor sharp device to puncture the skin).
– Most monitors measure the glucose concentration in whole blood (not plasma)
– The readings may vary by as much as 15% from samples submitted to the laboratory.
– Hand-held meters are reasonably accurate. If a reading seems unusual or does not match the clinical signs, a second reading should be taken or another method used to measure blood glucose.
– All blood glucose machines are least accurate during episodes of hypoglycemia (low blood sugar).
· Collecting a drop of blood from the ear (pinna) or a carpal pad or a footpad and analyzing this using a test strip. The test strip changes colour according to how much glucose is in the blood. After a short incubation period the colour change on the test strip should be compared with the colour chart on the accompanying packaging.
· A number of new methods are being tested. These include continuous, less invasive methods of glucose testing such as using a glucose sensor that can be placed under the skin that can record several hundred blood glucose levels over a two- to three-day period.
The results of these tests should be recorded and kept in a record book or on a chart.
Blood glucose curves
This is a series of blood glucose measurements made after a dose of insulin is administered. It is the most effective way to monitor insulin therapy and the only way to determine how the insulin is working in a given individual. Assumptions about the time to peak insulin action and the duration of action are often inaccurate.
To make a standard glucose curve, blood samples are taken just before and every 1.5 to 2 hours after insulin administration ideally until the next insulin dose is administered.
This is not always possible and an alternative is to take blood samples every 1.5-2 hours until the nadir blood glucose concentration has been reached. After this a further one or two blood samples should be taken at 1.5-2 hour intervals to make sure the glucose concentrations are increasing and to determine the point that the renal threshold is passed (duration of action). This is best done by plotting the results as a graph.
1. To establish initial insulin protocol at the time of diagnosis
2. Once or twice daily in dogs
3. Dose
4. To monitor the degree of regulation
5. To assess problems with regulation; If the response to insulin therapy is poor, a plasma glucose curve should be plotted, and every diagnostic effort should be made to rule out other problems.
4. To rule in or out rebound hyperglycemia
This occurs when nadir blood glucose concentrations are <3.6 mmol/l (65 mg/dl). In response, the blood glucose concentration rises rapidly and may exceed 33 mmol/l (600 mg/dl), which appears as a HIGH reading on most blood glucose meters. This requires a full glucose curve.
There are a number of ways of assessing past blood glucose concentrations
· Fructosamine
· Glycosylated (glycated) haemoglobin
Fructosamine
Fructosamines are stable complexes of carbohydrates and proteins that are produced by an irreversible, nonenzymatic glycosylation of protein. Glucose has a greater affinity for albumin in dogs. Fructosamine measurements can be used to diagnose and monitor diabetes mellitus in dogs. A single measure of fructosamine indicates the average glucose concentration over the previous 1-2 weeks.
· Highly sensitive
· Specific
· Distinguishes hyperglycemic, non-diabetic patients from diabetics with chronic hyperglycemia
· Depends on the level and duration of serum glucose concentration and the rate of turnover of specific plasma proteins. In dogs, the half-life of albumin is approximately 8 days.
· Changes more rapidly than glycosylated haemoglobin concentrations
· Useful in evaluating longer-term control and owner compliance with the administration of insulin
The determination of fructosamine concentration in the blood is performed using an adapted, commercially available, automated, colorimetric nitroblue tetrazolium technique. This laboratory test is fast, reproducible, inexpensive, easily automated and precise.
Although fructosamine levels are useful for evaluating the overall control of diabetes mellitus and long-term glucose regulation, there are some limitations.
· Unable to detect short-term or transient abnormalities in blood glucose, e.g., transient daily episodes of hypoglycaemia and/or hyperglycemia. This requires serial blood glucose measurements
· Fructosamine increases in hyperproteinaemia (e.g. hyperalbuminaemia due to dehydration in dogs)
· Albumin and fructosamine concentrations are highly correlated in dogs. Dogs with hypoalbuminaemia also have a decreased fructosamine concentration (false negative)
Glycosylated haemoglobin
Glycosylated haemoglobin is produced by the non-enzymatic, irreversible binding of glucose to haemoglobin in erythrocytes. As the blood glucose concentration increases, the rate of hemoglobin glycosylation also increases. The specific fraction of glycosylated hemoglobin that is measured is HbA1c. This is reported as a percentage of hemoglobin that is in the glycosylated form. The glycosylation of hemoglobin is a gradual process and is not affected by acute or transient hyperglycemia in dogs.
Glycosylated (glycated) hemoglobin concentration can be used as a screening test for diabetes mellitus, as well as for the monitoring of glycemic control in treated diabetic animals.
Therefore, glycosylated hemoglobin is a viable alternative to fasting glucose measurements because it is unaffected by stress related or post-prandial hyperglycemia. Glycosylated hemoglobin determination also is useful in long term monitoring of diabetic patients over the previous 2-3 months. The binding of glucose and hemoglobin is irreversible, the affected senescent erythrocytes must be degraded before the glycosylated hemoglobin value will decrease. Glycosylated hemoglobin is not used routinely to monitor diabetic dogs.
· Test not widely available for these species
· Not the most effective test due to the relatively long erythrocyte lifespan life span (approximately 110 days in dogs).
· Less effective for short-term monitoring than fructosamine because hyperglycemia must be present for at least 3 weeks before increased HbA1c values are detectable
· Affected by haemoglobin concentrations: may be increased or decreased due to polycythemia or anemia (renal disease), respectively.

Diet and exercise
Diet and exercise need to be controlled as these affect the insulin requirements.
Dietary management is extremely important in the successful control of diabetes mellitus. Ideally the diet should be exactly the same every day and given at the same time and tit-bits should not be given to avoid fluctuations in blood glucose concentrations. If titbits are given, a slice of carrot is suitable – high fiber, low fat! Some general guidelines are described below. It is very important that the routine chosen should be:
· Suitable for the owner
· Minimise blood glucose fluctuations (post-prandial hyperglycaemia)
In dogs that are underweight, it is desirable that weight is gained (“ideal” body weight).
Very calorie dense diets should be avoided, especially those that are high in soluble carbohydrates. In dogs that are overweight, it is desirable that weight is lost (“ideal” body weight) in a controlled fashion. Most diets designed to promote weight loss are in fact high fibre diets.
Water should be available at all times and its reduced consumption indicates successful treatment of the diabetes mellitus.
The same amount of moderate exercise should be given every day. Wide variations in exercise will affect insulin requirements.
Dogs
Commercial diets produced for diabetic dogs are available from veterinary surgeons. These diets are ideal for diabetic dogs as they release glucose more slowly during the day and can result in a lower requirement for insulin.
· Meals are usually timed to coincide with maximum insulin action (to avoid hypoglycaemia), and specific quantities and types of food are advised.
· For dogs administered insulin once daily this is quite straightforward
§ The first small meal (e.g. one-quarter or one-third of the daily ration) is given prior to the morning insulin injection. This allows the owner to see that the dog is feeling well and eating normally before the insulin is given. When the dog is eating can provide a good opportunity to administer the insulin.
§ The second meal is usually given about 6-8 hours later (i.e. three-quarters or two-thirds of the daily ration)
· For dogs administered insulin twice daily this appears, at first, to be more complicated since the fourth meal will be in the middle of the night!
§ The first small meal (e.g. one-sixth of the daily ration) is given prior to the morning insulin injection. This allows the owner to see that the dog is feeling well and eating normally before the insulin is given. When the dog is eating can provide a good opportunity to administer the insulin.
§ The second meal is usually given about 6-8 hours later (i.e. one-third of the daily ration)
§ The third small meal (e.g. one-sixth of the daily ration) is given prior to the afternoon/evening insulin injection. Once again this allows the owner to see that the dog is feeling well and eating normally before the insulin is given.
§ The fourth meal is usually given about 6-8 hours later (i.e. one-third of the daily ration)
· However, in practice, particularly if commercial high fibre diets are fed, the blood glucose fluctuations are minimized and all that has to be ensured is that there is not a hypoglycaemic episode during the night. Thus, the following seems to be more appropriate in practice:
§ The first small meal (e.g. one-sixth of the daily ration) is given prior to the morning insulin injection. This allows the owner to see that the dog is feeling well and eating normally before the insulin is given. When the dog is eating can provide a good opportunity to administer the insulin.
§ The second meal is usually given about 6-8 hours later (i.e. three-sixths of the daily ration)
§ A third small meal (e.g. one-sixth of the daily ration) is given prior to the afternoon/evening insulin injection.
§ A fourth small meal (e.g. one-sixth of the daily ration) is given last thing at night
· High fibre diets have been shown to reduce the peak elevation of blood glucose that follows eating, high fibre diets should be especially formulated or chosen from available commercial preparations to ensure consistency of calorie content, and nutritional completeness.
· Most importantly the dog must attain optimum weight, this reduces insulin demand in obese animals.
Further information for owners is available on www.pet-diabetes.com
Further information for veterinary practitioners is available on www.caninsulin.com
