
Bovine Viral Diarrhoea (BVD)
Introduction
Bovine viral diarrhoea (BVD) is a costly infectious disease for the dairy and beef industries. Advances in the knowledge of disease transmission, diagnostic tools and the introduction of effective vaccines have enabled the control and eradication of the BVD virus from infected herds.
Infection with BVDV can have different clinical manifestations depending on the herd’s immune and reproductive status. Infertility or abortion, diarrhoea, Mucosal Disease and more serious manifestations of disease related to immonosuppression (e.g. respiratory signs) are common clinical signs and can be seen simultaneously in a herd.
To view a video animation on the disease and its control please click here.
Aetiology – Causes
The causative agent of BVD is bovine virus diarrhoea virus (BVDV). Two genotypes have been identified:
• BVDV-1, distributed worldwide
• BVDV-2, identified mainly in North America and occasionally in Europe.
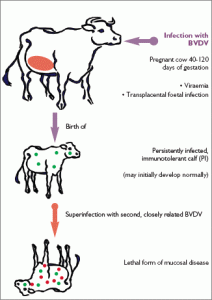
The BVD virus exists as two different biotypes: the noncytopathic (ncp) and the cytopathic (cp) biotypes. Only the ncp biotype can cause persistent infection of the bovine fetus. Calves persistently infected with an ncp BVD virus will develop Mucosal Disease if the virus mutates spontaneously into the cp biotype or the calf is infected with a cp virus closely related to its own ncp BVDV strain.
Epidemiology – Factors Influencing Disease Occurrence
BVD is most commonly introduced into the herd via persistently infected (PI) animals shedding the virus in a variety of secretions and excretions. The highest titre of virus is shed in saliva / respiratory secretions, though it can also be shed in faeces. As well as these methods, BVDV can also be transmitted via the introduction of a transiently infected animal or an animal containing an in-utero PI, via contaminated semen (from unapproved sources as licensed Irish AI stations must ensure semen is BVD-free), embryos (from unapproved sources that have not been subjected to adequate washing procedures), hypodermic needles or even contaminated rectal gloves. Sheep, goats and deer can act as reservoirs of infection.
A PI (carrier) is an animal that is shedding virus all of the time and cannot develop an immune response to either field virus or BVD vaccine. They arise due to an infection of the foetus inside a pregnant animal between approximately day 80-126 of pregnancy. At this time of pregnancy, the foetal immune system has not yet developed so the virus circulates in the bloodstream of the foetus persistently. The immune system subsequently develops and recognises the BVD virus as part of the calf similar to immune recognition of the calf’s eye, or ear etc. Therefore the virus can never be recognised as a foreign agent by these animals. The animal may die in utero or may be born totally normal. The dam of this calf meanwhile develops an immune response and clears the virus from her circulation. When this foetal calf is born and drinks colostrum, they become antibody positive only for a number of months though remain virus positive for life. These animals give problems for two reasons: they act as a source of virus infection for other animals, as they shed virus continuously, and secondly the strain that they are infected with may mutate to become more damaging. Since they cannot develop an immune response to BVD virus, disease becomes severe and is known as Mucosal Disease.

Clinical Signs
BVDV infection can result in a wide spectrum of clinical disease varying from sub-clinical infection to fatal disease.
Different clinical signs can be seen simultaneously in a herd. The most common clinical signs are:
• Infertility or abortion – foetal abnormalities can occur with infections later in pregnancy resulting in brain abnormalities – see photo – Calf with cerebellar hypoplasia unable to stand and “stargazing”
• Diarrhoea
• Mucosal disease – a condition that can vary in severity from mild to severe. Signs include ill-thrift, diarrhoea, ulceration in mouth and gastro-intestinal tract and lameness (from ulceration of feet). Death can ensue after a variable period of time
• Immunosuppression leading to respiratory signs or increase in severity of other diseases
From an economical aspect, the biggest losses from BVDV are from infection around the time of insemination and during pregnancy. The effects are dependent on the time of gestation that infection occurs.
Infection from approximately nine days prior to service until 120 days of gestation may result in:
• Failure to conceive
• Early embryonic death / abortion / congenital deformity
• Foetal loss
• Persistently infected calves

Diagnosis
reland began its BVD eradication scheme in January 2012, with a voluntary phase of testing calves for PI status throughout 2012. Since January 1st 2013 onwards, calves must be tested for BVD virus. Northern Ireland has operated its BVD eradication scheme in a similar manner one year later than the above timeline. BVD eradication in Ireland has focused on the elimination of PI’s. This can be achieved by ear-notch testing of young calves for BVD virus antigen. This approach also allows screening of cows. However, it is critical to ensure, without exception, that all calves are correctly matched to their dams. Under this scheme, calves are ear-notch sampled shortly after birth and the tissue assessed for the presence of BVD virus using a PCR test. If the calf is subsequently proven to be PI, then the dam should be tested for PI status by either blood sample or ear notch test for BVD virus.
BVD virus positive animals should be resampled 3 weeks after the initial sampling date to rule out transient infection.
Various other diagnostic options exist for BVD in herds. For further information on diagnosis in dairy herds, click here.
Control
Control of BVD is based on four equally important aspects:
- Elimination of PI’s – Those PI’s found under Diagnosis should be isolated and culled
- Biosecurity – Maintaining biosecurity involves avoiding introduction of animals into the herd and/or implementing stict isolation / quarantine of introductions until proven negative, and restricting access of livestock to external sources of infection e.g. double fencing is in place at all perimeters, considering carefully sources of biological materials such as embryos, semen etc.
- Vaccination – To eliminate PI’s is not sufficient alone to keep out infection and prevent it from continuing to spread. An additional control tool is to ensure animals are immune prior to becoming pregnant to prevent the formation of new PI’s. This is best achieved by the appropriate use of a BVD vaccine to prevent foetal infection during the danger period when PI’s are formed. Bovilis BVD is licensed for foetal protection. Ceasing BVD vaccination in the presence of a “clear” BVD herd test (no PI’s found) is a high-risk strategy for the re-introduction of BVD into your herd, as your herd becomes naive and open to infection soon after ceasing annual herd vaccination. Bovilis IBR Marker Live can now conveniently be mixed with Bovilis BVD and given on the same day for booster vaccination. To view a video demonstrating how to mix the vaccines please click here.
- Monitoring – This varies depending on the nature and risk status of your herd. Appropriate screening programmes can be discussed with your local veterinary practitioner.
Further information on the National BVD eradication program and BVD is available on the Animal Health Ireland website.
