An Overview of Neonatal Calf Diarrhoea
Neonatal calf diarrhoea (NCD), more commonly referred to as calf scour or neonatal enteritis, is a major health and welfare challenge on both dairy and suckler farms. NCD is a complex multifactorial disease involving a mix of inputs including the presence of diseases (and associated pathogens), unfavourable environmental conditions, nutrition and inadequate management. This article will highlight the prevalence of the disease in Ireland, the most frequently identified infectious agents, investigating cases and controlling the disease.
Neonatal enteritis is the most frequently diagnosed cause of mortality in calves up to one month of age with this condition comprising 25.9% of DAFM laboratory submissions diagnosed by post-mortem in 2020 in Ireland. Diagnoses of systemic infections and respiratory infections accounted for 21.1% and 10.5% respectively1 in this age group. The same report stated that 21% of the blood samples submitted were suboptimal for the transfer of immunoglobulins while 12% had FPT1. 50% of NCD cases occur in the first week of life. Diarrhoeic calves exhibit electrolyte disturbances, dehydration, and metabolic acidosis while depression and ataxia are frequently observed due to the accumulation of D-lactic acid.

High morbidity, treatment costs and mortalities associated with neonatal diarrhoea result in major economic losses. The sequelae of neonatal enteritis are not only limited to short-term effects but have significant impact on productivity. What are these significant consequences for the dairy and beef industries? Visible results might include reduced growth during the rearing period, due to reduced daily weight gain, a subsequent increase in the time to conception and an increase in the age at first calving for heifers2. No correlation has been established between the occurrence of diarrhoea in the first month of life and milk production in the first lactation2. However, heifer calves experiencing mild diarrhoea during the first 3 months of life have lower milk yields than heifers without diarrhoea3. Calves with severe cryptosporidiosis in the first 16 days of life have reduced weight gain for more than a 6-month period3.
Causative agents associated with neonatal diarrhoea can be classified as non-infectious or infectious. Non-infectious diarrhoea is mainly nutritional which develops due to either inadequate quality of milk replacers or poor management4. It is notable that increased intake of milk fed ad libitum does not result in a higher incidence of diarrhoeaversus conventional feeding5. Viruses, bacteria and parasites are pathogens implicated in infectious NCD. The relative frequency of enteropathogenic agents, identified in faecal samples of calves up to one month of age in 2020, are outlined in Figure 11. Rotavirus is the primary pathogen isolated from faecal samples, at a % prevalence of 32%. Rotavirus has consistently been the most frequently identified infectious agent in calf faecal samples, ranging from 30%-34% in the previous ten yearsin Ireland6. Cryptosporidia were identified in 14.4% samples and 11.4% had Campylobacter jejuni1. Co-infections with multiple agents frequently occur, exacerbating the severity of clinical signs observed and resulting in increased mortality6.
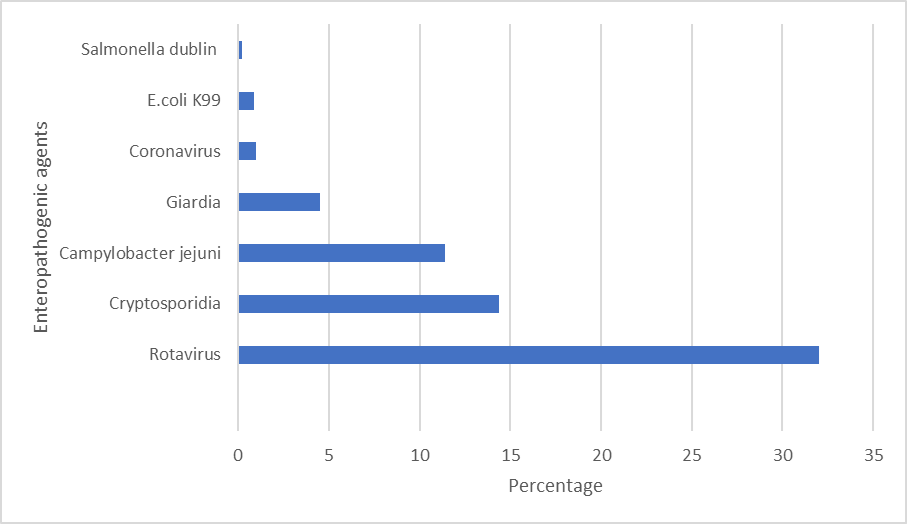
Rotavirus, from the Reoviridae family, is highly contagious and ubiquitous. It infects the enterocytes of the small intestine and stunts the villi resulting in undigested lactose facilitating bacterial proliferation7. There are three different mechanisms associated with rotaviruses resulting in diarrhoea; osmotic, secretory and malabsorptive. The primary route of transmission is faecal-oral while adult cattle are the initial source of infection for calves8. They present with scour from approximately 5 days of age, but typically up to 15 days. Rotavirus is the most frequently involved pathogen in mixed infections with a higher incidence of co- infection with Cryptosporidium versus enterotoxigenic E.coli (ETEC) and bovine coronavirus9.
Cryptosporidium parvum is a protozoan from the Cryptosporidiidae family. Osmotic and malabsorptive diarrhoea is observed due to mucosal changes in the ileum where there is stunting, swelling and fusion of the villi10. Transmission is via the faecal-oral route. Diarrhoea associated with C. parvum occurs from 5-28 days of age, typically during the second week of life. Infectious dose can be as low as 17 oocysts.
Campylobacter jejuni was isolated from 11.4% of samples tested in 2020. Campylobacter sp. are often present in the intestinal microbiota in cattle and are not considered pathogenic. C. jejuni is the most frequently isolated species11. 4.5% of faecal samples had the protozoan Giardia; it’s clinical significance in calves is equivocal.
Bovine coronavirus (BoCoV) was identified in 1% of samples submitted. Calves are most susceptible to infection with coronavirus from 1-3 weeks of age. BoCoV can also sporadically induce respiratory infections in calves. Coronavirus infects the epithelial cells of villi and crypts of both the small and large intestine. Cows are a source of infection as they can shed BoCoV-immune complexes in faeces12. Enterotoxigenic E. coli accounted for just under 1% of the agents isolated from faecal samples. Various antigenic determinants (pili) are used by E. coli to attach including F5(K99) and F41. It is important to ensure that antibodies induced by vaccination of the dam cover these antigens. ETEC typically causes scour in calves less than 3 days of age. Calves become rapidly dehydrated as the toxins produced by the bacteria cause secretion of water and electrolytes from the intestinal mucosa.
Investigation
- Obtain a thorough history
- Previous disease history – when, clinical signs, mortalities etc.
- Peripartum and colostrum management
- Age profiles
- Risk profiles – were calves bought-in and if yes, from how many sources
- Environmental conditions- bedding, drainage, draughts, disinfectants
- Treatments and outcomes
- Preventative strategies
- Results of previous diagnostic tests
- Establish causative agent. On-site testing kit can identify 4 pathogens. Take samples directly from 5 untreated early-stage cases. If there are any mortalities send to the laboratory for post-mortem as soon as possible for maximum diagnostic value as changes which occur are rapidly obscured by autolysis. (Some may suggest this has limited value as enteropathogens associated with NCD are common features of faecal samples from healthy calves; it is an important step in the investigation to ascertain if viral, bacterial, protozoal or co-infections exist to know how to address the disease).
- Evaluate both quality and quantity of colostrum intake; at least 50g/L of IgG antibodies (specific gravity >1.050 or ≥22 on BRIX measurement) and 10% of body weight respectively. Timing is also important and to promote the 3-2-1 rule to farmers is quite useful – Calves should get 3 litres of colostrum inside the first two hours of life for their first feed.
- Monitor for FPT; test serum samples in calves from 24 hours to 5 days of age. The ideal age to sample calves is from 2 days as peak circulation of immunoglobulins is achieved 36 hours post ingestion of colostrum1.
- Address management issues if present – peripartum management, colostrum management, biosecurity and vaccination.
Treatment
- Supportive care (an oral rehydration and buffering solution recommended when dehydration is < 8% in addition with a suckle reflex) and maintain temperatures within the thermoneutral zone (10-25⁰C for up to one month of age).
- Nutritional support (it is vital to maintain milk feeding as withdrawal will lead to malnourishment and ultimately weight loss13)
- Prevention of endotoxemia. Encourage farmers to always check all navels with a clean gloved hand for swelling or discharge inside the first few weeks of life. Ensuring bedding is adequate and dry improves risk profile.
- Antimicrobial treatment where appropriate (bacteraemic diarrhoeic calves are at risk of developing septicaemia, and could therefore benefit from administration of antimicrobials)
- Antiprotozoal treatment where appropriate
Control
Controlling NCD involves numerous components encompassing peripartum calving management, maximising the immunity of the calf, minimising the infectious pressure and reducing environmental stress. Implementation of these preventative strategies are critical to improve health and welfare of calves but will also help address issues such as the emergence of multidrug and pandrug resistant E. coli in faecal samples of calves with dairrhoea14.
Nutritional factors are not limited to the neonatal feeding regime but also to the feeding of the pregnant dam peripartum. Avoid overfeeding heifers to reduce incidence of dystocia and avoid straw diets for suckler cows prepartum as they can negatively affect immune status of the colostrum and consequently the calf’s immune status4.
To maximise immunity, provide agammaglobulinemic calves with good quality and quantity of colostrum within the first two hours of life. This will ensure passive immune transfer of immunoglobulins. Calves can be stomach tubed to ensure intake as colostrum quality decreases by 3.7% every hour after birth in tandem with the calf’s decreasing ability to absorb IgG after four to six hours after birth.
Calves’ immunity can be further enhanced by implementing a vaccination programme to provide active immunisation to pregnant heifers and cows to raise antibodies against E. coli adhesion F5 (K99) antigen and F41 antigens, rotavirus and coronavirus. For example, Bovilis Rotavec Corona, a trivalent vaccine, raises antibodies to reduce the severity of diarrhoea caused by both of these offending antigens of E. coli, reduce the incidence of scours caused by rotavirus and reduce the shedding of virus by calves infected with rotavirus or coronavirus.
Bovilis Rotavec Corona contains Bovine rotavirus inactivated Strain UK-Compton serotype G6P5, the most prevalent serotype15. Bovilis Rotavec Corona offers flexibility and convenience as once a vial is broached for the first time it may be used once more during the next 28 days. Calves from vaccinated dams are also considerably less likely to shed cryptosporidium parvum16. Vaccination can be incorporated into a control regime with halofuginone lactate during the first 7 days of life in the milk as a metaphylactic treatment against C. parvum. This two-step control programme can reduce excretion of enteropathogens and reduce the incidence of NCD17.
Exposure to enteropathogens is inevitable as they are ubiquitous. Nevertheless, by paying strict attention to hygiene, especially of calving pens, the load of enteric pathogens the neonatal calf is exposed to is decreased. Appropriate hygiene practices are crucial when caring for and treating diarrhoeic calves as many of these infectious agents have the potential to cause zoonotic disease.
NCD is undoubtedly a challenging clinical entity associated with significant morbidity and mortality. With prudent use of antimicrobials veterinary practitioners may incorporate adjuncts to the treatment protocols outlined above such as dietary supplementation of multispecies probiotic (MSP) or supplementation with bacteriophages. Supplementation with MSP can improve the average daily gain and promote intestinal epithelial cell growth18 while lytic bacteriophages supplemented to milk replacer may reduce the duration of scour resulting in less reduction of weight gain for the first 80 days of life19.
Understanding the pathogenesis and epidemiology of NCD on farms, veterinary practitioners can perform thorough investigations and devise a detailed farm specific control plan. Acting on the recommendations of the control plan will bolster the calves’ immunity and optimise their immune resilience particularly in the first month of life. Improving productivity and profitability will follow but the non-monetary benefits of improving calf welfare, reducing labour and consequently reducing stress for the famer are equally as important.
References
- All- Island Animal Disease Surveillance Report, 2020. Department of Agriculture, Food and Marine of Ireland, Agri-Food and Bioscience Institute and, Animal Health Ireland.
- Aghakeshmiri, F, Azzizzadeh, M, Farzaneh, N and Goriidooz, M. Effects of neonatal diarrhea and other conditions on subsequent productive and reproductive performance of heifer calves. Veterinary Research Communications, 2017, 41 (2): 107-112
- Pardon, B., Hostens, M., Duchateau, L., Dewulf, J., De Bleecker, K., Deprez, P. Impact of respiratory disease, diarrhea, otitis and arthritis on mortality and carcass traits in white veal calves. BMC Veterinary Research. 2013, 9, 79
- Lorenz, I, Mee, J.F, Earley, B and More, S.J. Calf Health from birth to weaning. I) General aspects of disease prevention. Irish Veterinary Journal, 2011, 64 (1)
- Jasper, J and Weary D.M. Effects of ad libitum milk intake on dairy calves. Journal of Dairy Science, 2002, 85 (11): 3054-2058
- Quinn, P.J. et al., Veterinary Microbiology and Microbial Disease. Blackwell Publishing. Chapter 62
- Geletu U.S, Usmael M.A and Bari F.D. Rotavirus in Calves and Its Zoonotic Importance. Veterinary Medicine International. 2021
- Brunauer, M, Roch, F and Conrady, B. Prevalence of Worldwide Neonatal Calf Diarrhoea Caused by Bovine Rotavirus in Combination with Bovine Coronavirus, Escherichia coli K99 and Cryptosporidium spp.: A Meta-Analysis. Animals : an Open Access Journal from MDPI, 2021, 11
- Taylor, M.A., Coop, R.L., and Wall, R.L. Veterinary Parasitology, 3rd Edition, Chapter 2: Parasites of Cattle
- Hansson, I, Tamminen, L.M, Frosth, S, Fernstrom, L.L, Emanuelson, U and Boqvist, S. Occurrence of Campylobacter spp. in Swedish calves, common sequence types and antibiotic resistance patterns. Journal of Applied Microbiology. 2021, 130 (6): 2111-2122
- Oma V.S, Tråvén M, Alenius S, Myrmel M and Stokstad M. Bovine coronavirus in naturally and experimentally exposed calves; viral shedding and the potential for transmission. Virology Journal. 2016;13(100)
- Heath S.E, Naylor J.M, Guedo B.L, Petrie L, Rousseaux C.G and Radostits OM: The effects of feeding milk to diarrheic calves supplemented with oral electrolytes. Canadian Journal of Veterinary Research. 1989, 53:477-485
- Feuerstein A, Scuda N, Klose C, et al. Antimicrobial Resistance, Serologic and Molecular Characterization of E. coli Isolated from Calves with Severe or Fatal Enteritis in Bavaria, Germany. Antibiotics (Basel). 2021;11(1):23.
- Recca, A. et al., Comparative Lactogenic Antibody Responses of Cattle from European Field Trials with a New Enteric Disease Vaccine. 2003, The Veterinary Record, 152(24) 751-752
- Trotz-Williams, L.A, Martin, S.W, Leslie, K.E, Duffield, T, Nydam, D.V. and Peregrine, A.S. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Preventative Veterinary Medicine, 2007, 82:12–28
- Meganck, V, Hoflack, G, Piepers, S and Opsomer,G. Evaluation of a protocol to reduce the incidence of neonatal calf diarrhoea on dairy herds. Preventative Veterinary Medicine, 2015, 118(1):64-70
- Yan-yan, W, Cun-xi, N, Chunsheng, X et al. Effects of dietary supplementation with multispecies probiotics on intestinal epithelial development and growth performance of neonatal calves challenged with Escherichia coli K99. Journal of the Science of Food and Agriculture, 2022, 102: 4373-4383
- Schmoeller, E, Matos A.D.C, Rahal N.M et al. Diarrhea Duration and Performance Outcomes of Pre-Weaned Dairy Calves Supplemented with Bacteriophage. 2021, Research Square
